This technical briefing note documents the current knowledge on Hepatitis E transmission routes and discusses the issues on the potential methods of response and prevention.
Hepatitis E Virus (HEV) is an emerging waterborne virus endemic in regions with poor/limited sanitation and hygiene, including large parts of Asia, Africa and South America.
The WHO estimates there are 3 million acute cases of HEV, and 56,600 HEV-related deaths per year (WHO 2014). HEV can lead from medium- to large-sized waterborne outbreaks, thereby causing a large proportion of cases with acute hepatitis. The most recent example is the HEV outbreak that has spread across South Sudan between 2012-14, with over 10000 cases and cross-border infection into neighboring countries; including 367 cases in South-Sudanese refugee camps in Ethiopia (UNHCR 2014). When such outbreaks occur, the humanitarian personnel in the area may have had little previous experience of handling this disease and its outbreaks. The consequent lack of preparedness can lead to much concern in the population, and unclear responses from field workers. As there are currently no curative therapies for Hepatitis E infection, prevention is the key intervention to limit the impact of this deadly disease. This technical brief uses current research on Hepatitis E to provide recommendations for prevention and how this can be translated into field actions for HEP E outbreak responses among WaSH teams. It aims to highlight best approaches for early detection and integration of preventative strategies into WaSH (and other) interventions. This brief does not provide an in depth discussion of the current research, however relevant research literature is cited within.
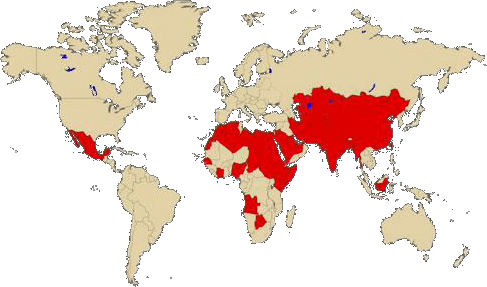
Fecal-oral route is the predominant mode of transmission of HEV, so clearly, measures aimed at proper treatment of drinking water, safe disposal of human excreta and improvement in personal hygiene are the keystones for its control. This document tries to address various issues related to prevent Hepatitis E outbreaks that may be of interest to WASH professionals.
Key facts of Hepatitis E Virus (HEV)
- HEV is a waterborne pathogen
- HEV causes an acute liver disease with a mortality rate of 2% in general population but 20-30% in pregnant women
- HEV has a long incubation period (4-10 w.)
- Natural immunity in humans after HEV- infection has been shown to be highly variable.
Hepatitis E Virus
Hepatitis E is usually an acute, self-limiting illness, similar in clinical presentation to hepatitis A. The clinical manifestations ofHEV can extend from asymptomatic infection, estimated in 73% of HEV infections,to acute viral hepatitis(17%)and acute liver failure (2%). Hepatitis E is distinguishable from other hepatitis due to a high attack rate in young adults and an increased morbidity and mortality in pregnant women. Symptomatic, infected pregnant women experience a case-fatality rate (CFR) as high as 20-30%, whereas the CFR in the general population is between 1 to 2% (Rein et al. 2012). The largest proportion of HEV infections globally are acquired by the fecal–oral route; however other routes have been described: parenteral transmission and vertical transmission from mother to child (Mirazo et al. 2014).
An incubation period of 4–10 weeks has been reported during Hepatitis E outbreaks in which the time of water contamination was known (Kumar et al. 2013).
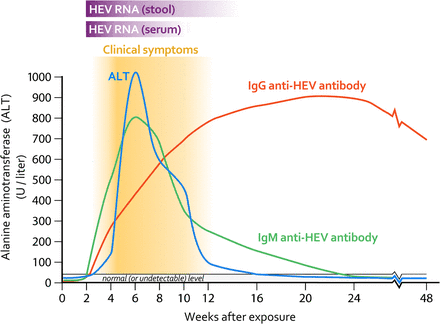
Excretion in stool of infective HEV by patients with acute hepatitis E generally persists for less than a month following symptom onset.
Once the disease is acquired (asymptomatic or symptomatic) the persistence of anti-HEV IgG and its ability to prevent reinfection by natural immunity in humans is highly variable and in some studies has been observed to be around 24 months(Krain et al. 2014).
Epidemiology
The epidemiology of Hepatitis E spans from sporadic or subclinical infections to large waterborne outbreaks with tens of thousands of cases.
Human genotype 1 and 2, found throughout Asia and North and Central Africa, has been the major cause of water-borne epidemics and significant sporadic disease whereas genotypes 3 and 4 are primarily zoonotic (wild and domestic pigs, deer and mongooses are reservoir of HEV) cause sporadic human disease.
Outbreaks are most common and frequent in tropical and subtropical regions and are usually separated by a few years. Such outbreaks have been observed in China, the Indian subcontinent, southeast and central Asia, the Middle East, and the northern and western parts of Africa. These outbreaks are usually large, and several hundred to several thousand persons are affected. Overall attack rates during Hepatitis e outbreaks range from 1% to 15%, with males outnumbering females in most of them (Kumar et al. 2013).
Evidence of fecal contamination of drinking water supplies has been associated with several outbreaks. Outbreaks occur most frequently during the rainy season, due to overflowing drains and the use of contaminated water for drinking (Mirazo et al. 2014). Moreover, several epidemiological and environmental studies in refugee camps have shown increased risk of transmission at household level. Studies during HEV outbreaks in South Sudan refugee camps described lack of obvious point-sources of infection and water storage contaminated by viruses at household level (Epicentre 2013). Moreover, a case-control study carried out in last HEV outbreak in Uganda has found major risk factors of infection in variable related to hygiene practices at household level (Teshale et al. 2010). Overall, findings suggest that, in outbreaks scenarios, transmission may be multi-factorial and include household level. However, the long incubation period of the disease hinders the identification of consistent risk factors or sources of infection.
Endemic areas & those at risk
Regarding the risk of an outbreak in refugee or internal displaced camps, it is very important to evaluate the risk and vulnerability of the displaced population considering endemicity of population origin and hosting area (check CDC map: Levels of endemicity for HEV)
When people migrate from a low into a high HEV- endemic area there is a greater risk to be impacted by an HEV outbreak due to the new exposure to this virus. For populations moving from an high into low HEV-endemic area, the risk also has to be considered due to the changes into the living conditions in the camps (density, water access, sanitation…) leading to a higher exposure and therefore higher transmission. Given the incubation period, this might not be visible immediately following the population afflux; however prevention measures should be implemented immediately.
Prevention measures
Shedding of enterically excreted HEV into the environment from infected patients plays a major role in HEV transmission. As stated, there is no specific treatment available; therefore the most effective method for prevention is access to clean water and sanitation and good hygiene practices
Monitoring, Control & Case Detection
In endemic areas or areas at risk, HEV incidence and mapping should be in place as soon as possible. Water quality surveillance, including viral indicators in drinking water sources, should be ongoing (Guerrero-Latorre et al. 2011). Furthermore, increased monitoring & precaution should be implemented with any new population movements or flooding events.
Sensitization, promotion & awareness raising activities should include key messaging related to the risks of HEV.
Hygiene promotion training for health workers should include the virus cycle, risks, warning signs and monitoring to improve prevention & early detection.
Water quality & treatment
The preventive measures need to focus primarily on ensuring supply of safe drinking water at household level:
- Chlorination of all sources of drinking water to ensure residual chlorine levels of 0.3–0.6 mg/l is the most adequate treatment as its effectivity has been recently evidenced in laboratory studies showing a 99% of HEV inactivation with at least 1 minute of contact time at a concentration of 0.41 mg/l (Girones et al. 2014).
- Reinforce household monitoring of residual chlorine to avoid water recontamination.
- Suggested UV treatment shows to be also effective towards HEV at laboratory level (data not published) but no residual effect implies less protection.
- Boiling has also been proved to be effective towards HEV, showing 99% reduction at 60ºC during 1h (Emerson et al. 2005)
- Promote the use of safe water storage containers at the household level to minimize recontamination
Sanitation
As with other viral outbreaks, prevention measures include reduction / elimination of open defecation and contamination of water sources. This may involve: clean up campaigns where lime is spread on the open defecation and it is removed: latrine construction programmes and the promotion of the use and maintenance of the toilets. Hand Washing after defecation and before eating also plays a critical role.
In areas endemic for HEV, there is a greater argument and need to emphasize the risks linked with open defecation and protection of groundwater sources from latrines (with a security radio of 30m), especially during raining season.
If there is a population influx, immediate action should be taken to ensure that newcomers have access to safe sanitation.
Hygiene Promotion campaigns
Hygiene training in endemic areas must include information on HEV, its pattern, early warning & best prevention.
Following any population movement in an endemic / high risk area, immediate precaution and monitoring should take place.
Considering the major risk of fatality in pregnant women, there should be a strong effort on both providing information and the opportunity for discussion with this highly vulnerable group. Working with health and antenatal care actors and women’s group should be envisaged.
Ensuring that access to safe water and sanitation is available is the key. Given the risk of poor water handling at household level, while on site water treatment should be ongoing, it is also advisable to provide water treatment at household level.
Sensitization for proper household water treatment (include water storage), handwashing and proper food preparation and handling should be emphasized.
Furthermore, ensuring that households have access to clean water storage options (jerrycans) is necessary to ensure that water is safe from collection to ingestion.
KEY PREVENTION MEASURES
- Evaluate the risk of new settlements in endemic areas
- Monitoring water quality (viral indicators)
- Ensure water treatment at the source: chlorination is the most adequate treatment, although UV and boiling can also be used
- Avoid open defecation
- Reinforce hygiene messages for correct water storage, hand washing and food handling
- Protect major risk group: pregnant women
References
CDC Hepatitis E Information for Health Professionals. Page last updated: September 17, 2012
Emerson S. et al 2005. Thermal Stability of Hepatitis E Virus. Journal Infect Diseases. doi: 10.1086/432488
Epicentre. Hepatitis E Outbreak in Yusuf Batil refugee camp Maban County, Upper Nile State, South Sudan, 2012-13. Mediciens Sans Frontieres 2013.
Girones R. et al 2014. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. Journal of Water and Health. In Press. doi:10.2166/wh.2014.027
Guerrero-Latorre, L. et al., 2011. Occurrence of water-borne enteric viruses in two settlements based in Eastern Chad: analysis of hepatitis E virus, hepatitis A virus and human adenovirus in water sources. Journal of water and health. doi: 10.2166/wh.2011.126.
Krain JL. et al 2014. Host Immune Status and Response to Hepatitis E Virus Infection. Clin. Microbiol. Rev. doi: 10.1128/CMR.00062-13
Kumar, S. et al., 2013. Hepatitis E virus: the current scenario. Inter. journal of infectious diseases. doi: 10.1016/j.ijid.2012.11.026
Mirazo, S. et al., 2014. Transmission, diagnosis, and management of hepatitis E: an update. Hepatic medicine : evidence and research, doi: 10.2147/HMER.S63417.
Rein, D.B. et al., 2012. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology (Baltimore, Md.). doi: 10.1002/hep.25505
Teshale EH et al 2010. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clinical Infectious Diseases. doi: 10.1086/651077.
UNHCR 2014. Field Brief: Hepatitis E Response In Refugee Settings.
UNHCR 2014. UNHCR steps up measures to rein in Hepatitis E among South Sudanese refugees in Ethiopia. Briefing Notes, 8 August 2014
WHO 2014 .Hepatitis E. Fact sheet n°280. Updated June 2014.
